Typical Mistake & Deficiencies During Construction of New Pharmaceutical Facilities
发布日期:2018-08-27
Foshan RuiTong Consulting Co.,Ltd. (Abbreviated as RuiTong, http://www.gdruitong.cn/) is a GMP consulting and execution company. Based on the regulatory requirements and our actual experience, RuiTong hereby summarizes the following contents for construction of new pharmaceutical facilities:
n Why talk about construction of new pharmaceutical facilities?
n Typical mistake & deficiencies during construction of new pharmaceutical facilities
n Correct project flow chart for constructing new pharmaceutical facilities
n Key successful factors for constructing new pharmaceutical facilities
Above-mentioned contents are described as follows:
1. Why talk about construction of new pharmaceutical facilities?
Because:
Ø Time to market today is a key factor to become profitable with a pharmaceutical product.
Ø Increasing number of competitors forces new makers to be quick and efficient.
Ø More and more complex production processes require high competence and deep process knowledge.
Ø Increasing regulatory requirements lead to more formal procedures (e.g. qualification and validation).
Ø Inspections by customers and authorities require a high level of compliance right from beginning.
Ø Design and construction of a GMP plant is time and money consuming and includes complex activities requiring a high level of attention.
Ø Design and construction of a GMP plant should follow well defined and formalized steps, which assure compliance with GMP regulation and allows it to operate as a fast track project at the same time.
2. Typical mistake & deficiencies during construction of new pharmaceutical facilities
Typical mistake & deficiencies during construction of new pharmaceutical facilities will normally result in violating GMP and registration requirements and delaying the project. Typical mistake & deficiencies are as follows:
Ø Quality awareness of top and middle management is missing, hence will result in critical deficiencies, wasting resources and delaying project, such as no market target, no URS for critical equipment and facilities, no design drawings for construction.
Ø Missing or bad overall (GMP) project management at the manufacturer (missing experience).
Ø Engineering tasks are seen as activities separated from GMP tasks.
Ø When starting an engineering project, the quality department of pharmaceutical company is usually not very involved.
Ø Vendors are not qualified according to supplier audit procedure, it is difficult for vendor to provide equipment satisfied with process and GMP requirements and to provide necessary documents (such as qualification of weldor, test reports of roughness).
Ø Qualification activities are started too late, when design is found to be not correct, it is difficult to be changed or more costs should be spent for changes and the project is delayed.
Ø Vendors are not sufficiently involved in qualification activities because qualification activities are not required during procurement, such as for DCS system.
Ø The documents had no uniform format, no document number. For example, FAT and SAT documents are not defined with uniform format, therefore can not be used later on for saving qualification activities.
Ø Common deficiencies are not considered into the design of documents and plant, missing the following activities during construction:
u Investigation of Anomalies.
u Quality tools application, such as CAPA, risk assessment, change control.
u Design and maintenance of equipment & premises.
Ø No URS for critical equipment or facilities, that means,no specification for DQ, IQ, OQ, PQ.
Ø The technical details of design and construction of facilities in the clean room are not corresponding with GMP requirements, such as slope is considered during pipelines installation, the certificates of materials of gaskets & lubricants are missing, quality of welding is not checked.
Ø Qualification protocols are not detailed and reasonable, such as acceptance criteria of qualification items are not clearly predefined, P&ID is not checked during IQ and P&ID is not updated to be the latest version, even there is no P&ID.
Ø Quality critical systems and procedures should be validated, but no risk assessment to define what are critical and what could be omitted.
3. Correct project flow chart for constructing new pharmaceutical facilities
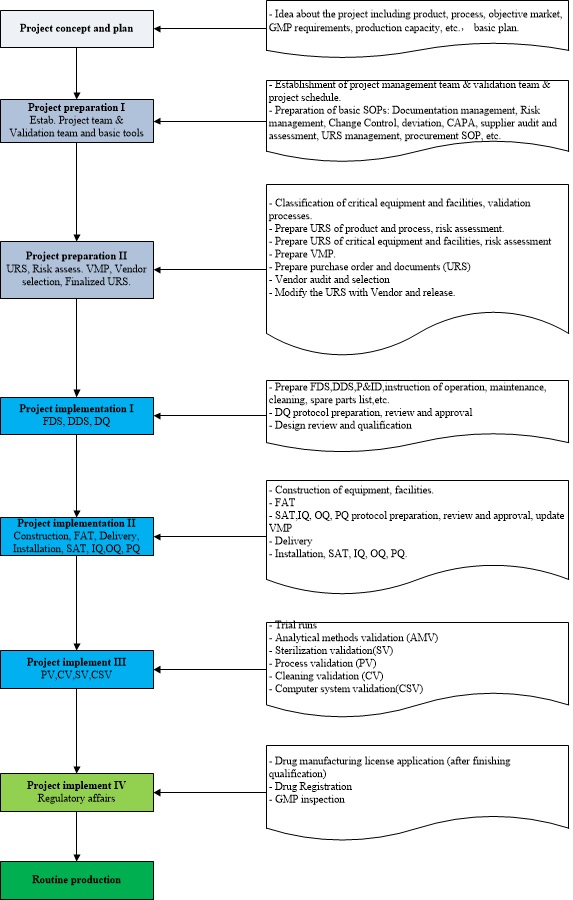
4. Key successful factors for constructing new pharmaceutical facilities
Ø Well defined project schedule including GMP activities.
Ø Well established master documents and procedures right in time, such as:
u Documentation management procedures
u Training management procedures
u Validation Master Plan (VMP)
u Qualification and validation procedures
u Risk management procedure
u Quality tools: Change control, deviation, CAPA…..
u URS management
u ……
Ø Well defined workflow for the Design, Engineering, Procurement.
Ø Preparation of URS of process, critical equipment and facilities, RA.
Ø Thorough selection of vendors.
Ø Training for involved staffs and vendors on different stages.
Foshan RuiTong Consulting Co.,Ltd. (Abbreviated as RuiTong) is a GMP consulting and execution company located in Foshan City, Guangdong Province, China. Its main services include GMP consulting and execution, DMF/CEP/ASMF registration and CFDA Drug registration, audit. Looking forward to your cooperation sincerely.
Foshan RuiTong Consulting Co.,Ltd.
Room 803, Fuwei Building, Huangqi GuangFo Road 1, Nanhai District, Foshan City, Guangdong Province, China
Website: http://www.gdruitong.cn/
Tel.: +86-136 6005 7841, +86-13695153512
E-mail: ruitong01@gdruitong.cn


