Review of Drug Project for One US Company
发布日期:2023-02-22
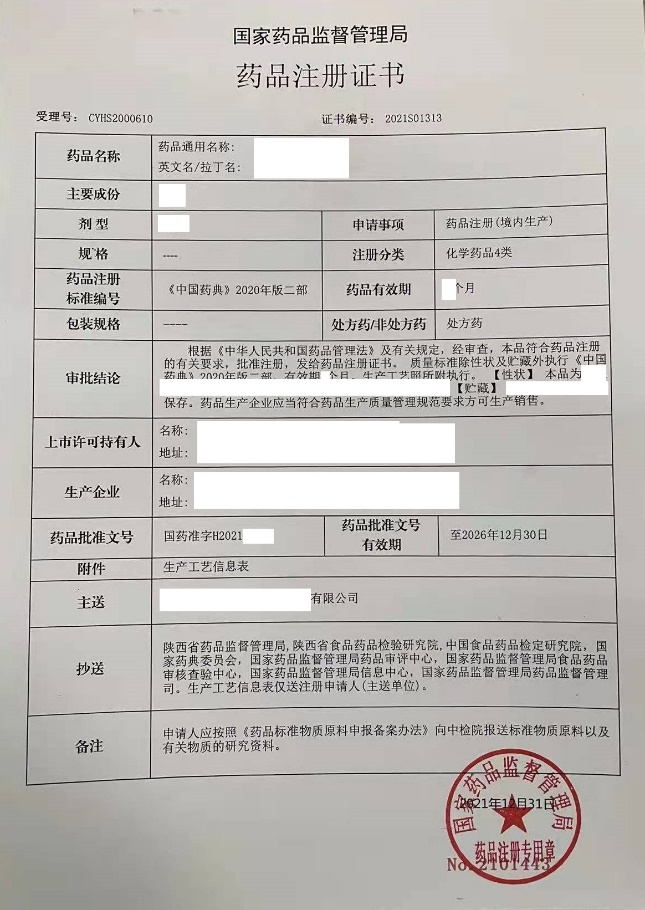
US company Shaanxi Co.,Ltd.(afterwards abbreviated as US company as confidential), located in Shaanxi province, is one pharmaceutical company newly set up for manufacturing drug dosage form. Foshan RuiTong Consulting Co.,Ltd. (afterwards abbreviated as RuiTong) supported US company for establishing quality system, drug registration, GMP compliance inspection from January, 2020 to February 2023. US company had obtained drug registration certificate grated by NMPA on December 31, 2021. US company had been inspected by Shaanxi Medical Products Administration on February 08-10, 2023, 3minor deficiencies were observed and no major and critical deficiencies were observed, US company had successfully passed the GMP compliance inspection.
 Foshan
RuiTong Consulting Co.,Ltd. is a GMP consulting and execution company located
in Foshan City, Guangdong Province, China, its main service contents include
GMP consulting and execution, DMF/CEP/ASMF/WHO PQ/China Drug registration, Audit.
RuiTong had provided the following services during drug project for US company:
Foshan
RuiTong Consulting Co.,Ltd. is a GMP consulting and execution company located
in Foshan City, Guangdong Province, China, its main service contents include
GMP consulting and execution, DMF/CEP/ASMF/WHO PQ/China Drug registration, Audit.
RuiTong had provided the following services during drug project for US company:
n Gap analysis and corrective actions schedule
n GMP documentation system
n Equipment qualification
n Validation (Process Validation, DCS system Validation, Analytical Methods Validation)
n GMP training
n GMP certification support and follow up
n Drug registration
RuiTong throughout insists vision with “Customer Focus, Result oriented” and help customer do every detail well really. If you need to know more about our services, please do not hesitate to contact us.
Foshan RuiTong Consulting Co.,Ltd.
Room 803, Fuwei Building, Huangqi, GuangFo Road No.1, Nanhai District, Foshan City, Guangdong Province, China
Website: http://www.gdruitong.cn/
Tel.: +86-13660057841, +86-13695153512
E-mail: ruitong01@gdruitong.cn


